
Interactive Feature Viewer
Last compiled: 08 September 2025
feature_viewer.Rmdsemla’s Feature Viewer is an interactive application
that can be run directly from R which makes it possible to interactively
visualize features present in a Seurat object and it also
makes it possible to manually annotate spots.
Some of the functionality is similar to what you can do with the Loupe
Browser or the ST viewer but if
you are using Seurat (or any other R package) for your
analyses, it can be cumbersome to import/export results back and forth
from R.
Having the option to interactively explore an SRT data set can be very useful. For example, a common task is to manually annotate spots based on morphological features of an H&E image which can only be done through an interactive application. Moreover, when we map gene expression to our H&E images in R, it is often difficult to associate their patterns with small morphological structures. At a quick glance, the expression of marker genes associated with small structures might appear random in plots, and it is only when we zoom in at the H&E image that we can see the tissues and cells that they are associated with.
Feature viewer
Using our mouse brain data set as an example, we’ll go through how to
use the FeatureViewer() application.
se <- readRDS(system.file("extdata/mousecolon", "se_mcolon", package = "semla")) |>
LoadImages()## ## ── Loading H&E images ──## ## ℹ Loading image from /private/var/folders/z8/nrcst881607gn95xh3_4qvb00000gp/T/RtmpAdeL2K/temp_libpath109e41827971/semla/extdata/mousecolon/spatial/tissue_lowres_image.jpg## ℹ Scaled image from 541x600 to 400x444 pixels## ℹ Saving loaded H&E images as 'rasters' in Seurat object
ImagePlot(se)
Data export
To initiate the viewer, we call the FeatureViewer()
function with our Seurat object as input.
FeatureViewer() will automatically attempt to export all
necessary files to a temporary directory (default) or a specified
directory (datadir). These files include tiled H&E
images, spot coordinates and an additional meta data file with image
related information. If you are working with a larger data set with
multiple tissue sections, you probably want to avoid exporting these
files each time you open the viewer. You can find more instruction
further down in this tutorial under Advanced.
Running the interactive viewer
When the app is closed, any changes made in the app will be returned
to our Seurat object, so we need to call
se <- FeatureViewer(se) to save changes to our
Seurat object. It is important to note that the application
should be exited by clicking on the ‘save’ icon in the
top right corner of the app, otherwise any changes that we’ve made will
not be returned to R.
NB: Like most functions provided in semla,
FeatureViewer() only works with Seurat objects
processed with semla.
se <- FeatureViewer(se)The app should open in your default browser:
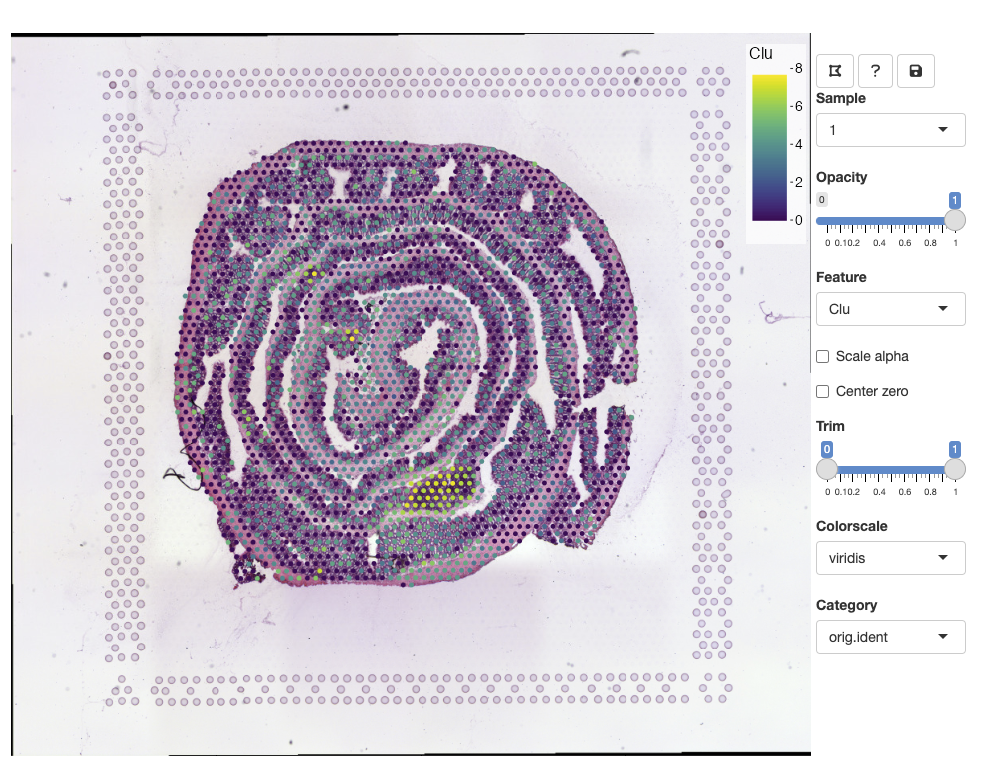
To get started, you can click on the ‘question mark’ icon which will give instructions on how to use the app:
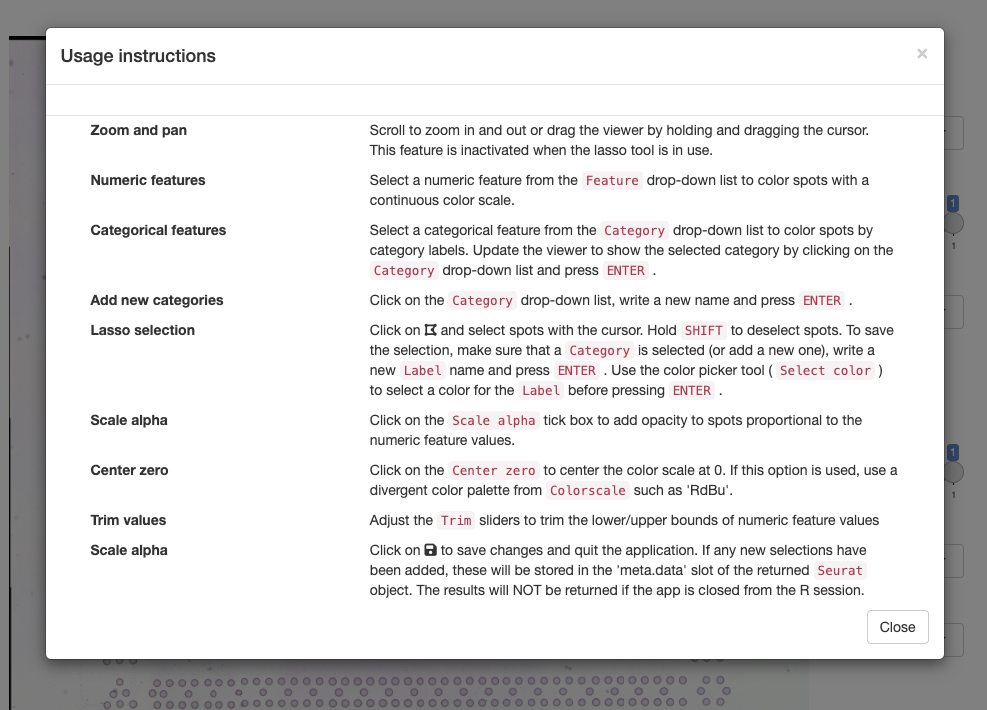
Zoom and pan
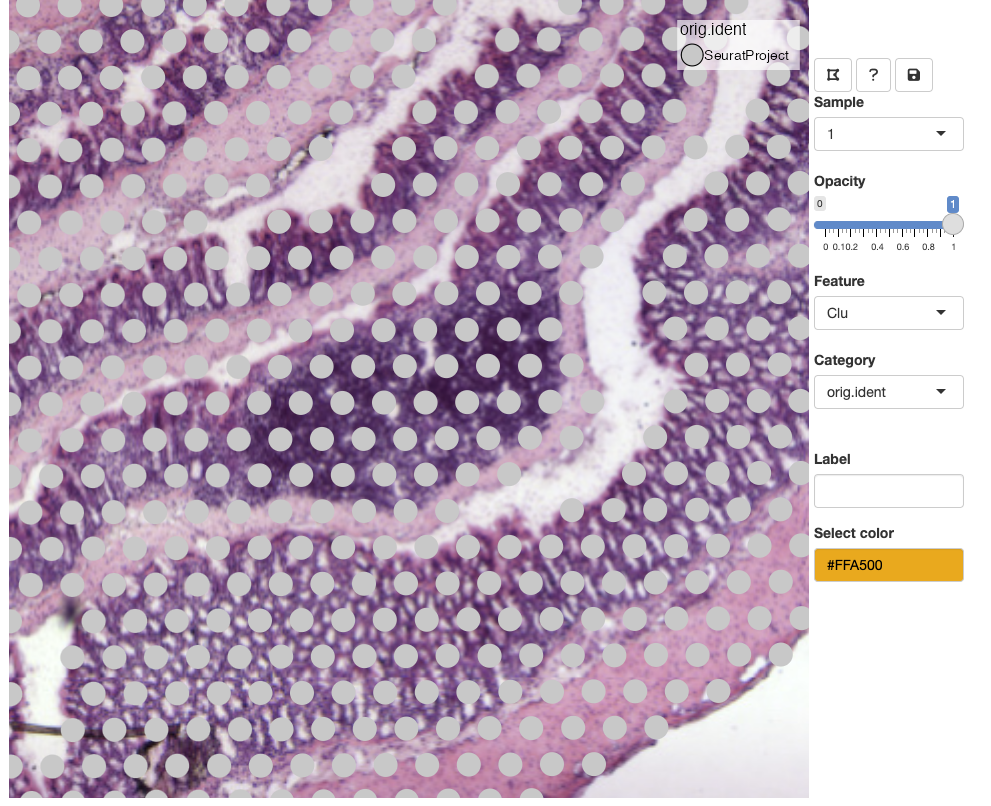
You can zoom in and out using the mouse wheel and pan by dragging clicking and dragging the H&E image. The opacity slider allows us to hide spots if we wish to view clear the view of the H&E image:
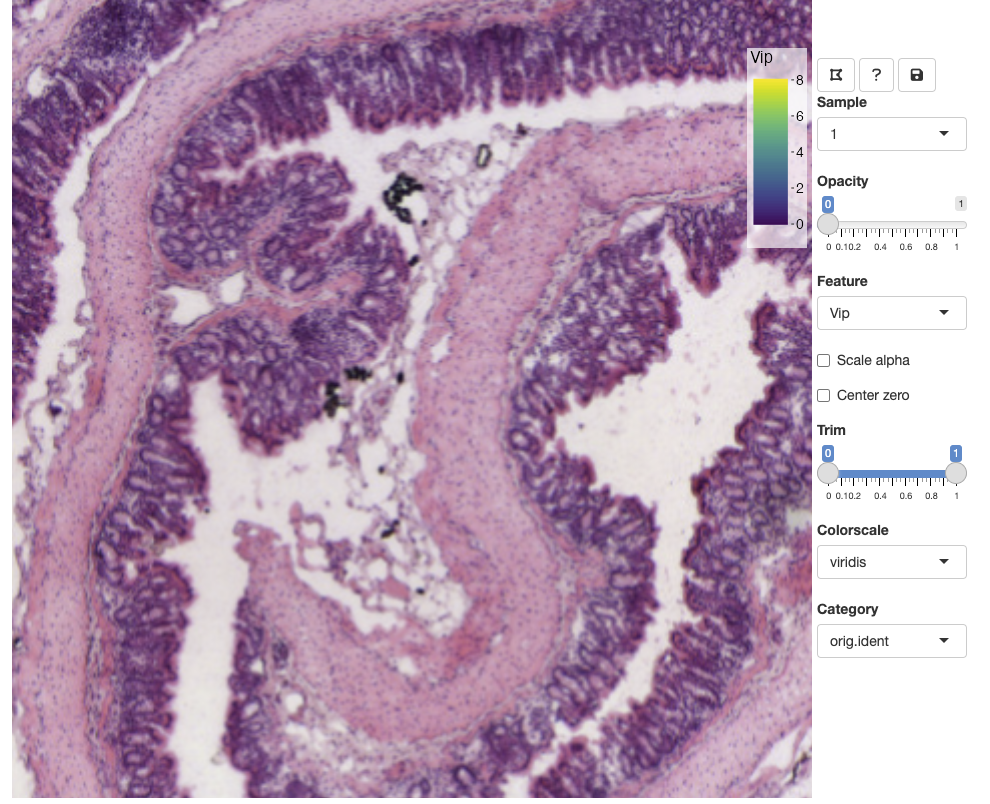
Selecting features
From the Feature drop down list, we can select any numeric feature
available in our Seurat object. This includes vectors
stored in the meta.data slot as well as dimensionality
reduction vectors stored in the reductions slot. If you
have multiple assays available in your Seurat object, only
features from the active assay will be available. This is to avoid
naming conflicts as different assays can share identical feature IDs. If
you want to select raw or scaled counts, you can specify what
Assay slot to use with the slot argument in
FeatureViewer.
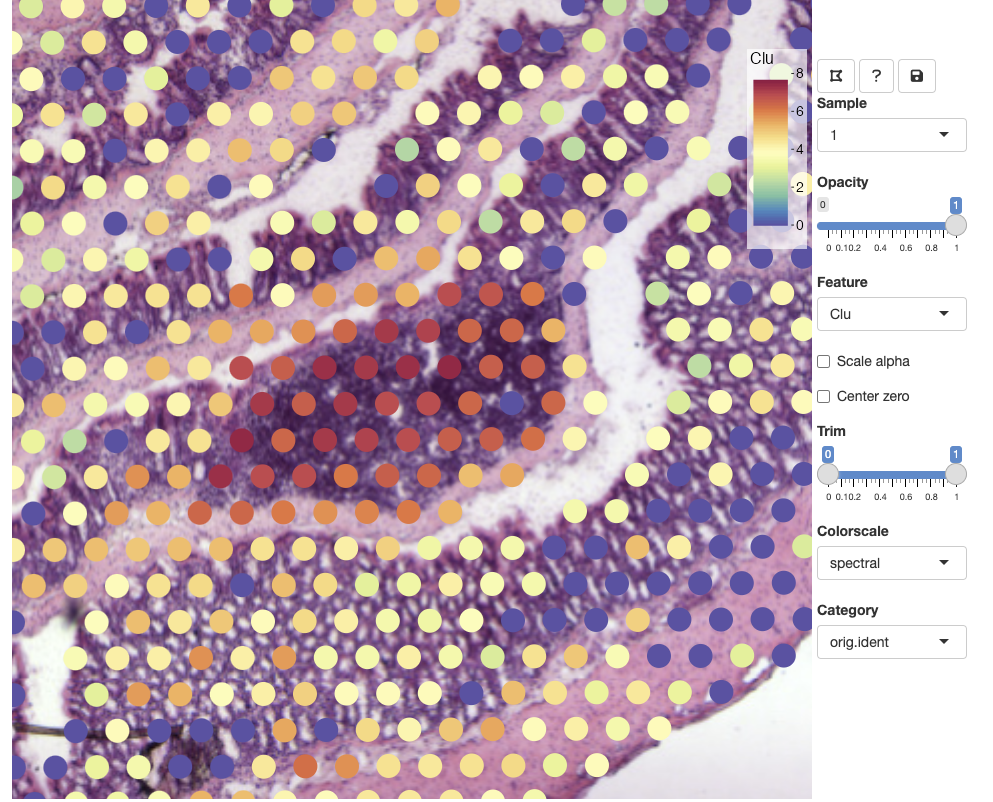
Other options
opacity slider: scale spot opacity.
scale alpha: scale the spot opacity of numeric features based on feature values.
Trim slider: trim lower and upper bounds of the selected numeric feature values. 0 corresponds to the minimum value and 1 corresponds to the maximum value.
Colorscale: select a color palette for numeric features.
center zero: centers the color scale at 0 which can be useful when visualizing centered values, for example principal component vectors or scaled expression. If center zero is active, it is appropriate to use a divergent color palette from ‘Colorscales’, such as
RdBu.
Category
Categorical variables stored in the Seurat object
meta.data slot are made available through the ‘Category’
drop-down list. You can select a new category or select the current
category and press ENTER to update the view.
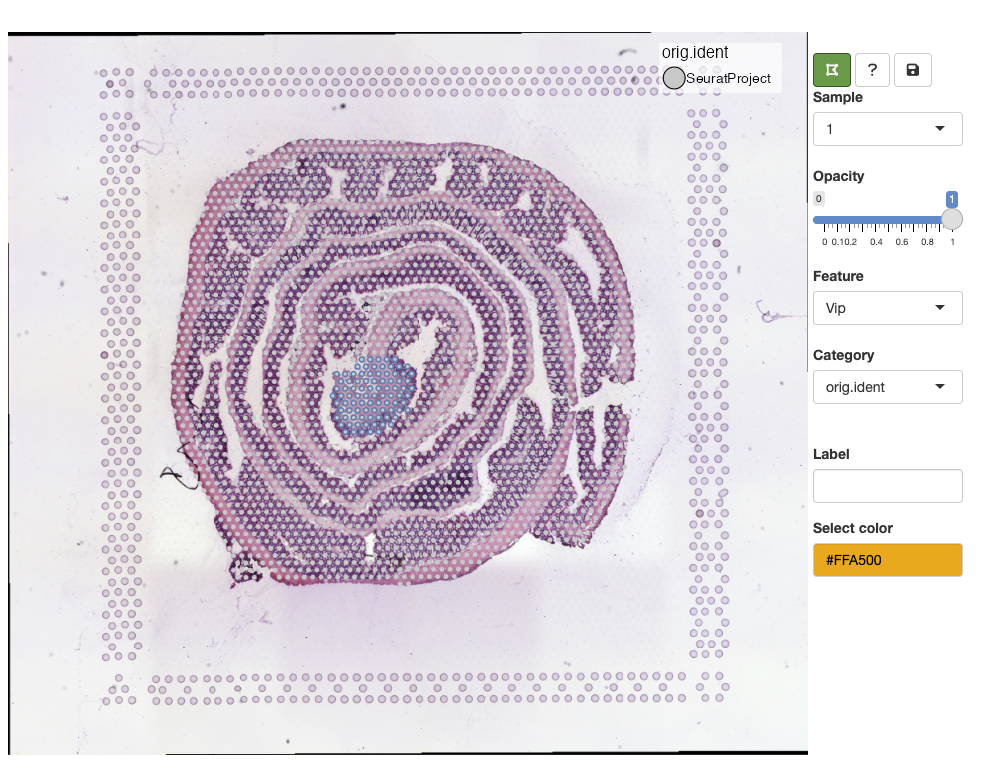
Lasso tool
In the example above, we selected the meta.data column
called orig.ident which currently only contains one label.
When a categorical variable is selected, we get the option to add new
labels. Before we can add a new label, we need to select spots with the
‘lasso tool’ in the top left corner of the toolbar:
Select a color from the color picker, write a new label name and
press ENTER to save the changes:
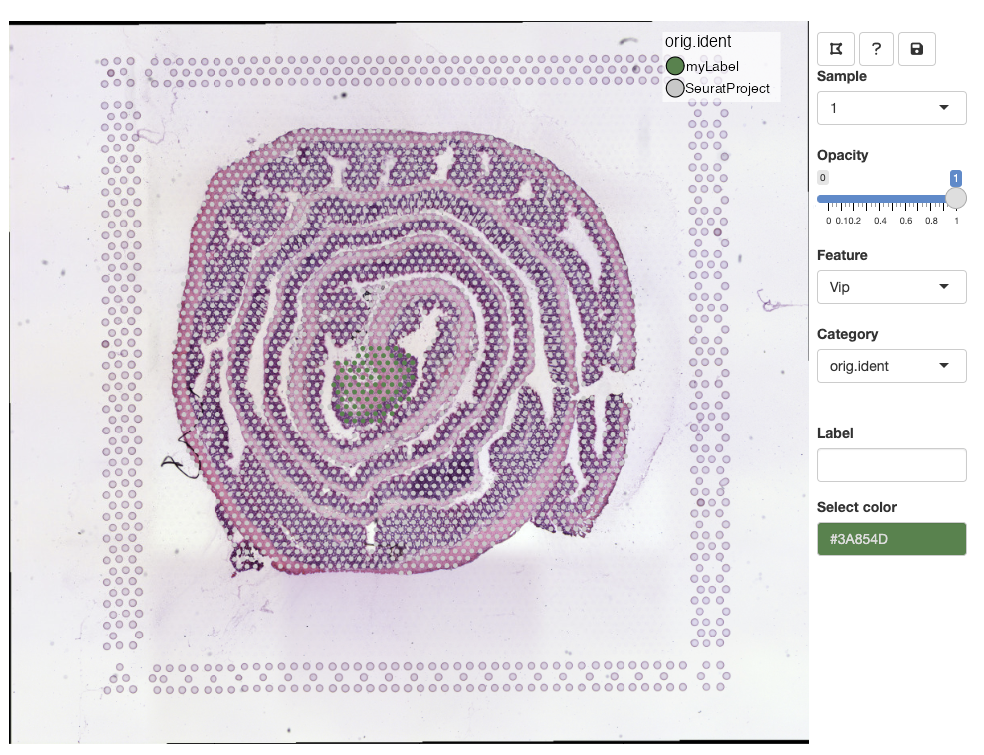
Once a new label has been added, it should pop up in the color legend.
Labels can share the same color, but if you wish to update the color
of an existing label, you can select spots from the same selection,
select a new color, write the same label name and press
ENTER. This will update the color for all spots with that
label.
NB: The lasso tool will freeze the current view, meaning that you will not be able to zoom and pan while the lasso tool is active. Deactivate the lasso tool to enable navigation in the viewer.
Advanced
Export data
You export all necessary data for the viewer to a specific output
directory with the ExportDataForViewer() function. This can
come in handy when you are working with larger datasets and you don’t
want re-export data every time you run the viewer. The output directory
is specified with the outdir argument which needs to be an
existing writable directory. If the files already exist in this
location, you will need to set overwrite=TRUE if you want
to overwrite the files.
ExportDataForViewer() creates a new directory inside
outdir called ‘viewer_data’, so the full path will be
FeatureViewer. You can also specify what samples to
export data for with the sampleIDs argument. For example,
if you chose to export one section 1 (sampleIDs=1), you will only be
able to view this section with FeatureViewer.
datapath <- ExportDataForViewer(se, sampleIDs = 1, outdir = "~/Downloads/")Now we can pass datadir = datapath to
FeatureViewer and it will load files from that directory
instead of exporting the files again.
se <- FeatureViewer(se, datadir = datapath)Multiple sections
When using multiple datasets, you will be able to select them from
the Sample drop-down list in the viewer. Note that when
working with many datasets, the app might slow down considerably. In
this case, it might be better to view only a few selected sections
specified with sampleIDs.
se_mbrain <- readRDS(system.file("extdata/mousebrain", "se_mbrain", package = "semla"))
se_mcolon <- readRDS(system.file("extdata/mousecolon", "se_mcolon", package = "semla"))
se_merged <- MergeSTData(se_mbrain, se_mcolon) |> LoadImages()
se_merged <- FeatureViewer(se_merged)Feature Viewer and docker
The viewer uses image tiles to create interactive maps of tissues
which are accessed from a static file server hosted locally. By default,
the server is hosted on 127.0.0.1:8080 and the viewer can
fetch the image tiles directly from this address. When using RStudio
Server via docker, we need to make sure that the port we are using
(inside the container) is exposed outside the container, otherwise the
tiles will not be accessible to the viewer which will be blank.
In the docker run ... command used to start the
container, we set -p 3030:3030 to expose port 3030 inside
the container to port 3030 outside the container. Now we should be able
to run the Feature Viewer with:
FeatureViewer(..., host = "0.0.0.0", port = 3030)Package version
-
semla: 1.4.0